Chelation-induced diradical formation as an approach to modulation of the amyloid-β aggregation pathway†
Abstract
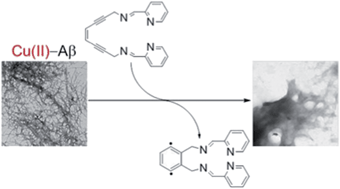
Current approaches toward modulation of metal-induced Aβ aggregation pathways involve the development of small molecules that bind metal ions, such as Cu(II) and Zn(II), and interact with Aβ. For this effort, we present the enediyne-containing ligand (Z)-N,N′-bis[1-pyridin-2-yl-meth(E)-ylidene]oct-4-ene-2,6-diyne-1,8-diamine (PyED), which upon chelation of Cu(II) and Zn(II) undergoes Bergman-cyclization to yield diradical formation. The ability of this chelation-triggered diradical to modulate Aβ aggregation is evaluated relative to the non-radical generating control pyridine-2-ylmethyl-(2-{[(pyridine-2-ylmethylene)-amino]-methyl}-benzyl)-amine (PyBD). Variable-pH, ligand UV-vis titrations reveal pKa = 3.81(2) for PyBD, indicating it exists mainly in the neutral form at experimental pH. Lipinski's rule parameters and evaluation of blood–brain barrier (BBB) penetration potential by the PAMPA–BBB assay suggest that PyED may be CNS+ and penetrate the BBB. Both PyED and PyBD bind Zn(II) and Cu(II) as illustrated by bathochromic shifts of their UV-vis features. Speciation diagrams indicate that Cu(II)–PyBD is the major species at pH 6.6 with a nanomolar Kd, suggesting the ligand may be capable of interacting with Cu(II)–Aβ species. In the presence of Aβ40/42 under hyperthermic conditions (43 °C), the radical-generating PyED demonstrates markedly enhanced activity (2–24 h) toward the modulation of Aβ species as determined by gel electrophoresis. Correspondingly, transmission electron microscopy images of these samples show distinct morphological changes to the fibril structure that are most prominent for Cu(II)–Aβ cases. The loss of CO2 from the metal binding region of Aβ in MALDI-TOF mass spectra further suggests that metal–ligand–Aβ interaction with subsequent radical formation may play a role in the aggregation pathway modulation.

 Please wait while we load your content...
Please wait while we load your content...