Electrochemical study of a nonheme Fe(ii) complex in the presence of dioxygen. Insights into the reductive activation of O2 at Fe(ii) centers†
Abstract
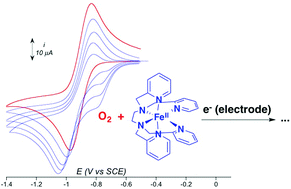
Recent efforts to model the reactivity of iron oxygenases have led to the generation of nonheme FeIII(OOH) and FeIV(O) intermediates from FeII complexes and O2 but using different cofactors. This diversity emphasizes the rich chemistry of nonheme Fe(II) complexes with dioxygen. We report an original mechanistic study of the reaction of [(TPEN)FeII]2+ with O2 carried out by cyclic voltammetry. From this FeII precursor, reaction intermediates such as [(TPEN)FeIV(O)]2+, [(TPEN)FeIII(OOH)]2+ and [(TPEN)FeIII(OO)]+ have been chemically generated in high yield, and characterized electrochemically. These electrochemical data have been used to analyse and perform simulation of the cyclic voltammograms of [(TPEN)FeII]2+ in the presence of O2. Thus, several important mechanistic informations on this reaction have been obtained. An unfavourable chemical equilibrium between O2 and the FeII complex occurs that leads to the FeIII-peroxo complex upon reduction, similarly to heme enzymes such as P450. However, unlike in heme systems, further reduction of this latter intermediate does not result in O–O bond cleavage.

 Please wait while we load your content...
Please wait while we load your content...