Studying the active-site loop movement of the São Paolo metallo-β-lactamase-1†
Abstract
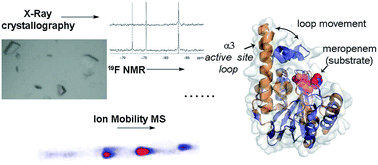
Metallo-β-lactamases (MBLs) catalyse the hydrolysis of almost all β-lactam antibiotics. We report biophysical and kinetic studies on the São Paulo MBL (SPM-1), which reveal its Zn(II) ion usage and mechanism as characteristic of the clinically important di-Zn(II) dependent B1 MBL subfamily. Biophysical analyses employing crystallography, dynamic 19F NMR and ion mobility mass spectrometry, however, reveal that SPM-1 possesses loop and mobile element regions characteristic of the B2 MBLs. These include a mobile α3 region which is important in catalysis and determining inhibitor selectivity. SPM-1 thus appears to be a hybrid B1/B2 MBL. The results have implications for MBL evolution and inhibitor design.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners

 Please wait while we load your content...
Please wait while we load your content...