Photoisomerization-induced gel-to-sol transition and concomitant fluorescence switching in a transparent supramolecular gel of a cyanostilbene derivative†
Abstract
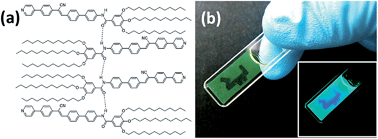
We report a transparent, fluorescent gel assembled from a cyanostilbene-containing material (compound 1), which incorporates bulky and flexible spacers via an amide group and exhibits aggregation-induced enhanced emission (AIEE). The photoinduced morphological change (gel-to-sol) upon the photoisomerization of 1 was successfully demonstrated, which was accompanied by a fluorescence color switching from the aggregate emission (490 nm) to the monomer emission (465 nm). It is notable that the photoisomerization of the cyanostilbene moiety in our innovative system induces both the gel-to-sol transition and the fluorescence color.

 Please wait while we load your content...
Please wait while we load your content...