Electronic infrared light absorption of a tri-palladium complex containing two π-expanded tetracene ligands†
Abstract
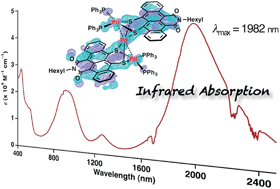
A large π-electron conjugated system consisting of three bridging palladium metals and two π-expanded tetracene derivatives was synthesized to have a narrow HOMO–LUMO gap for long-wavelength light absorption. The product, Pd3(TIDS)2(PPh3)4 (TIDS = tetracene imide disulfide), showed far long-wavelength light absorption reaching the infrared region (absorption maximum = 1982 nm, ε = 4.0 × 104 M−1 cm−1 in CH2Cl2 solution; 2500 nm in the solid state). X-ray crystallography revealed a tri-metallic structure composed of three square-planar Pd coordination planes. The total oxidation number of the three Pd atoms is +4. Quantum chemical calculations were used to elucidate wholly delocalized π-conjugation in the non-coplanar structure and the HOMO–LUMO transition for this unique absorption band. Time-resolved transient absorption measurements revealed the excited state dynamics characterized by a triplet charge transfer state lifetime of 400 ps and a λmax of 1280 nm. A mononuclear Pd complex, Pd(TIDS)(PPh3)2, was also synthesized as a reference compound, and characterized with spectroscopic and X-ray crystallographic analyses.

 Please wait while we load your content...
Please wait while we load your content...