Novel ortho-OPE metallofoldamers: binding-induced folding promoted by nucleating Ag(i)–alkyne interactions†
Abstract
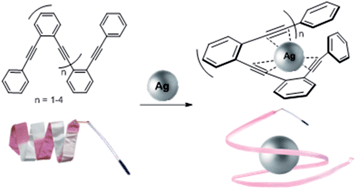
We have developed a new family of ortho-oligophenylene ethynylene (o-OPE) metallofoldamers. The folding of these helicates is induced by nucleating carbon–metal interactions between Ag(I) cations and the alkynes of the inner core of the o-OPEs. These o-OPEs form metal–organic assemblies where at least three alkyne moieties are held in close proximity to form novel Ag(I)-complexes with the metal ion lodged into the helical cavity. NMR titration experiments and photokinetic studies have provided quantitative data about the thermodynamic and kinetic features of such binding/folding phenomena. X-ray diffraction and DFT studies have been performed to extract structural information on how the Ag(I) cation is accommodated into the cavity. The great simplicity and versatility of these new metallofoldamers open up the possibility to develop novel structures with applications in material science and/or in asymmetric catalysis.


 Please wait while we load your content...
Please wait while we load your content...