Hydrophenylation of ethylene using a cationic Ru(ii) catalyst: comparison to a neutral Ru(ii) catalyst†
Abstract
Charge neutral Ru(II) complexes of the type TpRu(L)(NCMe)Ph [Tp = hydridotris(pyrazolyl)borate; L = CO, PMe3, P(OCH2)3CEt or P(O![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) CH2)2(OC
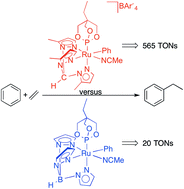
CH2)2(OC![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) CH3)] have been previously reported to catalyze the hydrophenylation of ethylene (Organometallics, 2012, 31, 6851–6860). However, catalyst longevity for the TpRu(L)(NCMe)Ph complexes is inhibited by competitive ethylene C–H activation. For example, ethylene C–H activation limits catalysis using TpRu(P(OCH2)3CEt)(NCMe)Ph to a maximum of 20 turnover numbers for conversion of benzene and ethylene to ethylbenzene. In contrast, reaction of the cationic Ru(II) complex [(HC(pz5)3)Ru(P(OCH2)3CEt)(NCMe)Ph][BAr′4] [HC(pz5)3 = tris(5-methyl-pyrazolyl)methane; BAr′4 = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate] (0.025 mol% relative to benzene) in benzene with C2H4 (15 psi) at 90 °C gives 565 turnover numbers of ethylbenzene after 131 hours. The production of 565 turnovers of ethylbenzene corresponds to an approximate one-pass 95% yield with ethylene is the limiting reagent and is a 28-fold improvement compared to the charge neutral catalyst TpRu(P(OCH2)3CEt)(NCMe)Ph. Under identical conditions, the activity of [(HC(pz5)3)Ru(P(OCH2)3CEt)(NCMe)Ph][BAr′4] is only 1.3 times less than TpRu(P(OCH2)3CEt)(NCMe)Ph, but the increased stability of the cationic Ru(II) catalyst allows reactivity at much higher temperatures (up to 175 °C) and significantly enhanced rates.
CH3)] have been previously reported to catalyze the hydrophenylation of ethylene (Organometallics, 2012, 31, 6851–6860). However, catalyst longevity for the TpRu(L)(NCMe)Ph complexes is inhibited by competitive ethylene C–H activation. For example, ethylene C–H activation limits catalysis using TpRu(P(OCH2)3CEt)(NCMe)Ph to a maximum of 20 turnover numbers for conversion of benzene and ethylene to ethylbenzene. In contrast, reaction of the cationic Ru(II) complex [(HC(pz5)3)Ru(P(OCH2)3CEt)(NCMe)Ph][BAr′4] [HC(pz5)3 = tris(5-methyl-pyrazolyl)methane; BAr′4 = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate] (0.025 mol% relative to benzene) in benzene with C2H4 (15 psi) at 90 °C gives 565 turnover numbers of ethylbenzene after 131 hours. The production of 565 turnovers of ethylbenzene corresponds to an approximate one-pass 95% yield with ethylene is the limiting reagent and is a 28-fold improvement compared to the charge neutral catalyst TpRu(P(OCH2)3CEt)(NCMe)Ph. Under identical conditions, the activity of [(HC(pz5)3)Ru(P(OCH2)3CEt)(NCMe)Ph][BAr′4] is only 1.3 times less than TpRu(P(OCH2)3CEt)(NCMe)Ph, but the increased stability of the cationic Ru(II) catalyst allows reactivity at much higher temperatures (up to 175 °C) and significantly enhanced rates.

 Please wait while we load your content...
Please wait while we load your content...