Unusual orientation and reactivity of alkyl halides in water-soluble cavitands†
Abstract
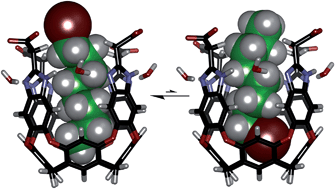
Water-soluble deep cavitands with ionic peripheral groups take up hydrophobic guest compounds in D2O. Longer n-halides and α,ω-dihalides C7–C11, are bound and NMR is used to deduce their arrangements within the cavitand. The guests tumble rapidly on the NMR timescale, but unexpectedly, the halogen atoms compete with CH3 groups for the aromatic panels near the bottom of the cavitand. Even a thiol can be found in the depths of the cavitand but not hydroxyl groups: 1,12-dodecanediol folds in the space leaving the ends exposed to solvent. The hydrolysis of bound guests is accelerated when the halides are near the cavitand rim.

 Please wait while we load your content...
Please wait while we load your content...