TMP (2,2,6,6-tetramethylpiperidide)-aluminate bases: lithium-mediated alumination or lithiation–alkylaluminium-trapping reagents?†‡
Abstract
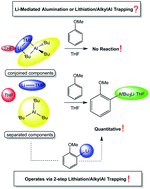
The lithium TMP-aluminate bases “LiTMP·Al(iBu)3” 1 and “LiTMP·Al(TMP)(iBu)2” 2, where TMP is 2,2,6,6-tetramethylpiperidide, have recently come under the spotlight as “aluminating” reagents in that they can perform aluminium–hydrogen exchange on a wide variety of aromatic substrates. Previous studies have intimated that 1 existed as a single species in THF solution formulated as [(THF)·Li(μ-TMP)(μ-iBu)Al(iBu)2] 1·THF, having a contacted ion pair structure as evidenced by an X-ray crystallographic study of isolated crystals. But here using anisole as a case substrate it is revealed that pre-crystallised 1·THF cannot deprotonate anisole at all whether in hexane or THF solution contradicting earlier in situ applications of 1 which revealed near quantitative metallation of anisole. NMR spectroscopic studies of 1 made in situ in THF solution ascribe this reactivity distinction from 1·THF to complex equilibria involving five major species in LiTMP·THF, Al(iBu)3·THF, [{Li(THF)4}+{Al(TMP)(iBu)3}−] 1·(THF)4, [(THF)·Li(μ-TMP)(μ-OC4H7)Al(iBu)2], 4, and (TMP)Al(iBu)2·THF. Reagent 2 in contrast is found to exist as only two separated homometallic species in LiTMP·THF and (TMP)Al(iBu)2·THF in THF solution. The constitutions of 1 and 2 in non-polar hexane solution are also revealed. With the aid of DFT calculations, discussion focuses on the fact that none of the aluminate species present in THF solutions of 1 or 2 can deprotonate/metallate anisole, instead the metallation processes appear to be LiTMP lithiations followed immediately by trapping by an alkylaluminium complex, in a metal exchange which drives the reaction to the product (arylaluminated) side.

 Please wait while we load your content...
Please wait while we load your content...