Stereoselective allylboration of imines and indoles under mild conditions. An in situ E/Z isomerization of imines by allylboroxines†
Abstract
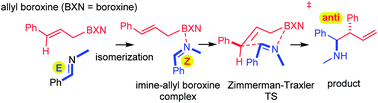
Direct allylboration of various acyclic and cyclic aldimine, ketimine and indole substrates was performed using allylboronic acids. The reaction proceeds with very high anti-stereoselectivity for both E and Z imines. The allylboroxines formed by dehydration of allylboronic acids have a dual effect: promoting E/Z isomerization of aldimines and triggering the allylation by efficient electron withdrawal from the imine substrate.

 Please wait while we load your content...
Please wait while we load your content...