Molybdenum–molybdenum quadruple bonds supported by 9,10-anthraquinone carboxylate ligands. Molecular, electronic, ground state and unusual photoexcited state properties†
Abstract
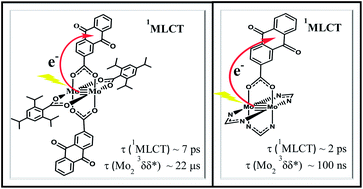
The preparation of trans-Mo2(TiPB)2(O2C-2-AnQ)2, I, and Mo2(DAniF)3(O2C-2-AnQ), II, where, O2C-2-AnQ = 9,10-anthraquinone-2-carboxylate, TiPB = 2,4,6-triisopropylbenzoate and DAniF = N,N′-di-(p-anisyl)formamidinate are described together with electronic structure calculations employing density functional theory on model compounds. Compounds I and II have been further characterized by 1H NMR spectroscopy, MALDI-TOF mass spectrometry, electrochemical studies, UV-vis-NIR and steady state emission spectroscopy, time-resolved (fs and ns) transient absorption and infrared spectroscopy. Compound II has been structurally characterized by a single crystal X-ray crystallography study. Both compounds show metal-to-ligand charge transfer transitions, 1MLCT, corresponding to Mo2 δ to L π* (L = O2C-2-AnQ) and have S1 states with relatively long lifetimes (τI ∼ 8 ps; τII ∼ 2 ps). Femtosecond time-resolved IR studies, fs-TRIR indicate that for I, despite its relatively small HOMO–LUMO gap, and II the negative charge in the S1 state resides on one anthraquinone carboxylate ligand. For II, fs-TRIR spectroscopy detects the Mo23δδ* state with τ ∼ 100 ns despite the presence of a lower energy 3MLCT state. This, together with earlier findings indicates a significant kinetic barrier to the interconversion of MLCT and Mo2 δδ* states.

 Please wait while we load your content...
Please wait while we load your content...