Sodium phosphaethynolate, Na(OCP), as a “P” transfer reagent for the synthesis of N-heterocyclic carbene supported P3 and PAsP radicals†
Abstract
Sodium phosphaethynolate, Na(OCP), reacts as a P− transfer reagent with the imidazolium salt [DippNHC–H][Cl] [DippNHC = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene] to give the parent phosphinidene–carbene adduct, DippNHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PH, with the loss of CO. In a less atom economic reaction, the cage compound, P7(TMS)3 (TMS = SiMe3) reacts likewise with the imidazolium salt to yield DippNHC
PH, with the loss of CO. In a less atom economic reaction, the cage compound, P7(TMS)3 (TMS = SiMe3) reacts likewise with the imidazolium salt to yield DippNHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PH thereby giving two entry points into parent phosphinidene-based chemistry. From the building block DippNHC
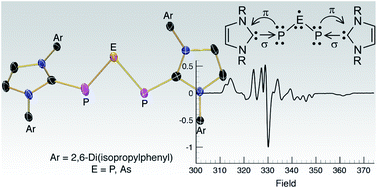
PH thereby giving two entry points into parent phosphinidene-based chemistry. From the building block DippNHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PH, the carbene-supported P3 cation [(DippNHC)2(μ-P3)][Cl] was rationally synthesized using half an equivalent of PCl3 in the presence of DABCO (1,4-diazabicyclo[2.2.2]octane). The corresponding arsenic analogue, [(DippNHC)2(μ-PAsP)][Cl], was synthesized in the same manner using AsCl3. The reduction of both [(DippNHC)2(μ-P3)][Cl] and [(DippNHC)2(μ-PAsP)][Cl] into their corresponding neutral radical species was achieved simply by reducing the compounds with an excess of magnesium. This allowed the electronic structures of the compounds to be investigated using a combination of NMR and EPR spectroscopy, X-ray crystallography, and computational studies. The findings of the investigation into (DippNHC)2(μ-P3) and (DippNHC)2(μ-PAsP) reveal the central pnictogen atom in both cases as the main carrier of the spin density (∼60%), and that they are best described as the P3 or PAsP analogues of the elusive allyl radical dianion. The phosphorus radical was also able to undergo a cycloaddition with an activated acetylene, followed by an electron transfer to give the ion pair [(DippNHC)2(μ-P3)][P3(C(COOMe))2].
PH, the carbene-supported P3 cation [(DippNHC)2(μ-P3)][Cl] was rationally synthesized using half an equivalent of PCl3 in the presence of DABCO (1,4-diazabicyclo[2.2.2]octane). The corresponding arsenic analogue, [(DippNHC)2(μ-PAsP)][Cl], was synthesized in the same manner using AsCl3. The reduction of both [(DippNHC)2(μ-P3)][Cl] and [(DippNHC)2(μ-PAsP)][Cl] into their corresponding neutral radical species was achieved simply by reducing the compounds with an excess of magnesium. This allowed the electronic structures of the compounds to be investigated using a combination of NMR and EPR spectroscopy, X-ray crystallography, and computational studies. The findings of the investigation into (DippNHC)2(μ-P3) and (DippNHC)2(μ-PAsP) reveal the central pnictogen atom in both cases as the main carrier of the spin density (∼60%), and that they are best described as the P3 or PAsP analogues of the elusive allyl radical dianion. The phosphorus radical was also able to undergo a cycloaddition with an activated acetylene, followed by an electron transfer to give the ion pair [(DippNHC)2(μ-P3)][P3(C(COOMe))2].

 Please wait while we load your content...
Please wait while we load your content...