Breaking conjugation: unusual regioselectivity with 2-substituted allylic substrates in the Tsuji–Trost reaction†
Abstract
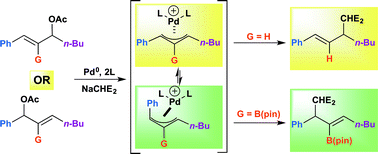
η3-Allyl palladium complexes are key intermediates in Tsuji–Trost allylic substitution reactions. It is well known that (η3-1-aryl-3-alkyl substituted allyl)Pd intermediates result in nucleophilic attack at the alkyl substituted terminus. In contrast, the chemistry of (η3-1,2,3-trisubstituted allyl)Pd intermediates is relatively unexplored. Herein we probe the regioselectivity with 1,2,3-trisubstituted allylic substrates in Tsuji–Trost allylic substitution reactions. DFT investigation of cationic (η3-1-Ph-2-B(pin)-3-alkyl-allyl)Pd(PPh3)2 intermediates predict that nucleophilic attack should occur preferentially on anti-allyls rather than the syn-isomers to generate benzylic substitution products under Curtin–Hammett conditions. Experimentally, systematic studies with 1,2,3-trisubstituted allylic substrates revealed that a Linear Free Energy Relationship (LFER) is observed when Charton steric parameters of the C-2 substituents are plotted against the log of the ratio of regioisomers. Bulkier C-2 substituents in 1,2,3-trisubstituted η3-allyl palladium intermediates provide stronger preference for nucleophilic attack at anti-oriented benzylic termini. Additionally, the geometry of 1,4-elimination products supports the presence of anti-allyl palladium intermediates.

 Please wait while we load your content...
Please wait while we load your content...