Transient electronic and vibrational absorption studies of the photo-Claisen and photo-Fries rearrangements†
Abstract
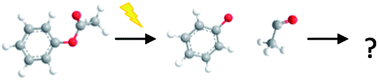
The liquid-phase photo-Claisen and photo-Fries rearrangement dynamics of allyl phenyl ether and phenyl acetate in cyclohexane solution have been interrogated via ultrafast transient absorption spectroscopy. Following excitation at 267 nm, the reaction progress is monitored on a picosecond time-scale by electronic and vibrational absorption spectra obtained from broadband UV/Visible and mid-infrared probe pulses. The evolution of the ground and excited electronic states of the parent molecule, the radicals produced by photo-induced homolytic bond fission, and intermediate cyclohexadienones formed via recombination of the produced radical pair are followed, providing new insight and detail on the reaction mechanisms. Subsequent kinetic analysis allows determination of rate coefficients as well as quantum yields for the processes involved. These examples serve to highlight the utility of employing broadband UV-Visible and infrared probe spectroscopies, in conjunction, to unravel the mechanisms of photochemical reactions in solution. The underlying photo-physics that initiates bond fission in this class of molecules is also addressed in the context of the role of dissociative (n/π)σ* excited states.

 Please wait while we load your content...
Please wait while we load your content...