A 115In solid-state NMR study of low oxidation-state indium complexes†
Abstract
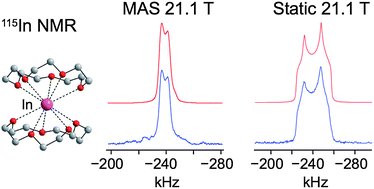
115In solid-state NMR (SSNMR) spectroscopy is applied to characterise a variety of low oxidation-state indium(I) compounds. 115In static wideline SSNMR spectra of several In(I) complexes were acquired with moderate and ultra-high field NMR spectrometers (9.4 and 21.1 T, respectively). 115In MAS NMR spectra were obtained with moderate and ultra-fast (>60 kHz) spinning speeds at 21.1 T. In certain cases, variable-temperature (VT) 115In SSNMR experiments were performed to study dynamic behaviour and phase transitions. The indium electric field gradient (EFG) and chemical shift (CS) tensor parameters were determined from the experimental spectra. With the aid of first principles calculations, the tensor parameters and orientations are correlated to the structure and symmetry of the local indium environments. In addition, calculations aid in proposing structural models for samples where single crystal X-ray structures could not be obtained. The rapidity with which high quality 115In SSNMR spectra can be acquired at 21.1 T and the sensitivity of the 115In NMR parameters to the indium environment suggest that 115In SSNMR is a powerful probe of the local chemical environments of indium sites. This work demonstrates that 115In NMR can be applied to a wide range of important materials for the purpose of increasing our understanding of structures and dynamics at the molecular/atomic level, especially for the characterisation of disordered, microcrystalline and/or multi-valence solids for which crystal structures are unavailable.

 Please wait while we load your content...
Please wait while we load your content...