The frustrated Lewis pair pathway to methylene phosphonium systems†
Abstract
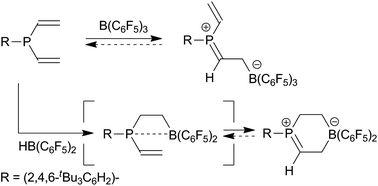
Reaction of HB(C6F5)2 with (tBu3C6H2)P(vinyl)2 does not yield the expected ethylene-bridged frustrated Lewis pair (FLP) but its cyclic methylene phosphonium isomer which is formed in a unique way by subsequent internal 1,2-P/B addition to the vinylphosphane unit. The product is a rare example of a stable phosphorus analogue of the ubiquitous iminium salts. It features a short P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (1.637(3) Å). Subsequent reactions with pyridine and with dihydrogen probably proceed by equilibration with the “invisible” ethylene-bridged FLP isomer. The reaction of (tBu3C6H2)P(vinyl)2 with B(C6F5)3 gave the zwitterionic acyclic methylene phosphonium product that was crystallized at low temperature and isolated. The X-ray crystal structure analysis also revealed a short P
C bond (1.637(3) Å). Subsequent reactions with pyridine and with dihydrogen probably proceed by equilibration with the “invisible” ethylene-bridged FLP isomer. The reaction of (tBu3C6H2)P(vinyl)2 with B(C6F5)3 gave the zwitterionic acyclic methylene phosphonium product that was crystallized at low temperature and isolated. The X-ray crystal structure analysis also revealed a short P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (1.635(5) Å).
C bond (1.635(5) Å).

 Please wait while we load your content...
Please wait while we load your content...