Probing liquid behaviour by helium atom scattering: surface structure and phase transitions of an ionic liquid on Au(111)†
Abstract
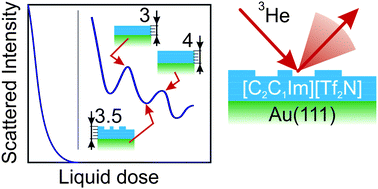
We demonstrate the application of the helium atom scattering technique to ionic liquid surfaces, specifically to study structure and phase transitions in layers of the ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide [C2C1Im][Tf2N] adsorbed onto the (111) surface of single crystal gold (Au) in UHV. We observe the formation of successive ordered layers of ion pairs during adsorption of [C2C1Im][Tf2N] and find that a dose equivalent to three layers of ion pairs is needed to wet the surface. The presence and position of the elastic diffraction peaks in helium diffraction scans indicates that there is registry between the surface layer of even thick adsorbed films (>8 layers or ∼36 Å) of [C2C1Im][Tf2N] and the Au(111) substrate, with the [C2C1Im][Tf2N] structure described by the matrix notation (3 2, −6 8). For substrate temperatures between 150 and 320 K, the intensity of specularly reflected helium and the diffraction peaks reveal a phase transition from a crystalline phase to a liquid, with melting occurring between 270 and 280 K, higher than in the bulk. We find no significant difference between scattering from the solid and liquid surfaces, and find a Debye–Waller factor for this system 2W ∼ 1.

 Please wait while we load your content...
Please wait while we load your content...