Rhodium-catalyzed regioselective opening of vinyl epoxides with Et3N·3HF reagent – formation of allylic fluorohydrins†
Abstract
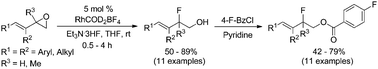
A highly regioselective rhodium-catalyzed ring-opening of vinyl epoxides with Et3N·3HF reagent to form branched allylic fluorohydrins is described. The reaction occurs at room temperature under ambient air and relies on RhCOD2BF4 as an effective catalyst, providing the desired 1,2-addition allylic fluorohydrins in moderate to good yields with excellent levels of regioselectivity. Mechanistic studies demonstrate that the regioselective ring-opening of enantiopure vinyl epoxide occurs with inversion of stereochemistry.

 Please wait while we load your content...
Please wait while we load your content...