Copper-catalyzed fluorination of 2-pyridyl aryl bromides†
Abstract
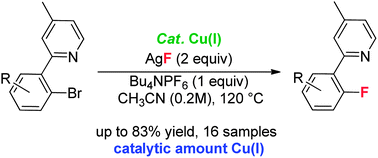
Copper(I)-catalyzed cross-coupling of aryl halides is the subject of extensive interest in synthetic chemistry, but the related catalytic fluorination is unsuccessful. Herein, we have developed a novel copper-catalyzed fluorination of aryl bromides using AgF as the fluorine source. In this transformation a pyridyl directing group is essential for successful catalytic fluorination. A XANES/EXAFS study indicated that the presence of the pyridyl group is essential to stabilize the Cu(I) species and accelerate oxidative addition of the aryl bromide. Further mechanistic studies implicated a Cu(I/III) catalytic cycle in this Cu(I)-catalyzed fluorination, and final aryl C–F bond formation possibly proceeds through an irreversible reductive elimination of the ArCu(III)–F species. This rare report of catalytic fluorination by a copper catalyst provides a valuable foundation for further development of Cu(I)-catalyzed fluorination of aryl halides.

 Please wait while we load your content...
Please wait while we load your content...