Conformation and reactivity in dibenzocyclooctadienes (DBCOD). A general approach to the total synthesis of fully substituted DBCOD lignansvia borostannylative cyclization of α,ω-diynes†
Abstract
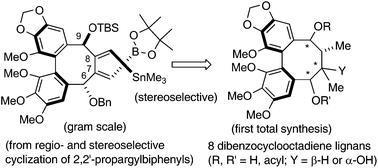
Dibenzocyclooctadienes (DBCOD) are a class of plant-derived natural products that exhibit a broad range of biological activities. These include cytotoxicity, anti-hepatitis-B activity, inhibition of HIV replication and of NO production and activity. None of the fully substituted DBCODs have been prepared before. 2,2′-Dipropargylbiphenyls undergo highly regio- and atropselective cyclizations mediated by a [B-Sn]-reagent, 1-trimethylstannyl-2,5-diazaborolidine (Me3Sn–B[–N(Me)CH2CH2(Me)N–], in the presence of Pd(II)-catalysts, to give highly functionalized DBCOD precursors. The configuration of the newly created, axially chiral, 1,2-bis-alkylidene moiety is controlled by the resident chirality of the starting

 Please wait while we load your content...
Please wait while we load your content...