Pd-catalyzed oxidative cross-coupling between two electron rich heteroarenes†
Abstract
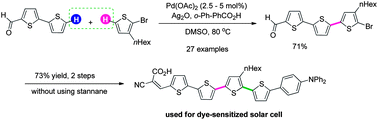
The transition-metal-catalyzed oxidative cross-coupling between two coupling partners with similar structure and electronic characteristics remains a challenge owing to difficulty in suppressing undesired homo-couplings. We herein report a Pd-catalyzed oxidative cross-coupling between two

 Please wait while we load your content...
Please wait while we load your content...