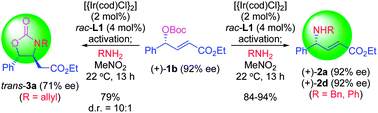
A pair of iridium-catalyzed regiospecific and stereospecific reactions of the carbonates of γ-hydroxy α,β-unsaturated esters were developed. The reaction pathways are strongly affected by the choice of amines employed. A diverse range of γ-substituted α,β-unsaturated γ-amino esters were prepared in excellent yields with various amine nucleophiles such as benzylamine, diallylamine, morpholine, aniline and N-methylaniline. Substitution at the γ-position was well tolerated, encompassing alkyl, aryl and heteroaryl substituents. Enantioenriched (E)-α,β-unsaturated γ-amino esters could also be synthesized from the corresponding enantioenriched allylic carbonates with complete chirality transfer. In sharp contrast, a series of 3,4-disubstituted oxazolidin-2-ones were obtained by using allylamine as a nucleophile.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...