Controlled modulation of the helical sense and the elongation of poly(phenylacetylene)s by polar and donor effects†‡
Abstract
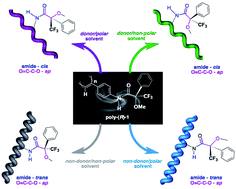
The elasticity (stretched/compressed) and helical sense (clockwise/anticlockwise) of a dynamic helical poly(phenylacetylene) (PPA), bearing ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )C–C(–O) and (H–)N–C(
)C–C(–O) and (H–)N–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). An increase in polarity shifts the conformational equilibrium of the (O
O). An increase in polarity shifts the conformational equilibrium of the (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )C–C(–O) bond in favour of the most polar synperiplanar (sp) conformer, that is more sterically demanding, thus forcing the
)C–C(–O) bond in favour of the most polar synperiplanar (sp) conformer, that is more sterically demanding, thus forcing the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) bond favouring the shift to the cis form of the
O) bond favouring the shift to the cis form of the

 Please wait while we load your content...
Please wait while we load your content...