Sequential and click-type postfunctionalization of regioregular poly(3-hexylthiophene) for realization of n-doped multiplet state†
Abstract
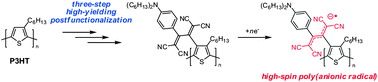
Regioregular poly(3-hexylthiophene) was postfunctionalized by a three-step high-yielding reaction producing an electron-accepting 1,1,4,4-tetracyanobuta-1,3-diene unit at the regioregular position of the thiophene rings. Electrochemical measurements of the postfunctionalized polythiophene derivative showed two one-electron

 Please wait while we load your content...
Please wait while we load your content...