Enhanced isosteric heat, selectivity, and uptake capacity of CO2adsorption in a metal-organic framework by impregnated metal ions†
Abstract
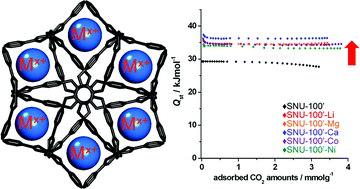
We demonstrate by experimental and theoretical studies that the impregnation of various metal ions such as Li+, Mg2+, Ca2+, Co2+, and Ni2+ in the pores of an anionic MOF, [Zn3(TCPT)2(HCOO)][NH2(CH3)2] (SNU-100′) significantly enhances isosteric heat, selectivity, and uptake capacity of the CO2 adsorption in the MOF. Due to the electrostatic interactions between CO2 and the extra-framework metal ions, the isosteric heats of CO2

 Please wait while we load your content...
Please wait while we load your content...