Large hydroazaacene diimides: synthesis, tautomerism, halochromism, and redox-switchable NIR optics†
Abstract
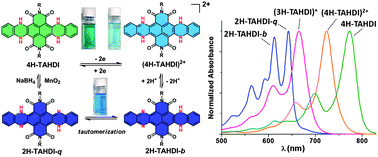
A series of dihydro- and tetrahydro-tetraazaacene diimides containing 6 or 7 laterally fused six-membered rings were synthesized. Halochromic and redox-switchable vis-NIR optical characteristics were exhibited. Quinonoid tautomers of dihydrotetraazaacene derivatives were obtained and characterized, which exhibited adequate stability and existed in equilibrium with the more commonly observed benzenoid tautomer.

 Please wait while we load your content...
Please wait while we load your content...