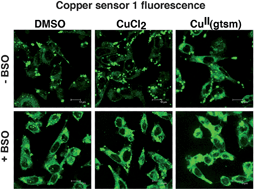
Copper is an essential biometal involved in critical cell functions including respiration. However, the mechanisms controlling its sub-cellular localization during health and disease remain poorly understood. This is partially due to the difficulty of detecting a metal ion that is bound tightly to metallo-chaperone and detoxification molecules in the cell. A BODIPY-based Cu fluorescent probe CS1 (Cu sensor 1) has been applied in innovative attempts to visualize monovalent Cu pools within cells (Zeng et al., J. Am. Chem. Soc., 2006, 128, 10–11). Inspired by this work, we sought to use CS1 to identify sub-cellular localization of Cu delivered to M17 neuronal or U87MG glial cells by a cell-permeable bis(thiosemicarbazonato)Cu(II) complex, CuII(gtsm). This complex increases cellular Cu concentrations by factors of 10–100 when compared to treatment with equivalent concentrations of CuCl2 (Donnelly et al., J. Biol. Chem., 2008, 283, 4568–4577). However, we were unable to identify any specific increase in CS1 fluorescence in neurons or glia treated with CuCl2 or with CuII(gtsm), despite controls revealing a large increase in total cellular Cu with the latter treatment. Further in vitro characterization of CS1 suggests that, consistent with its relatively weak affinity for CuI (KD ≈ 10−11 M), it is unlikely to compete with endogenous proteins with sub-picomolar affinities, nor with glutathione, the endogenous redox buffer essential for functional maintenance of many proteins, including those that bind CuI. Moreover, we show that CS1 is localized predominantly to lysosomes and that the observed background fluorescence may be attributed to increased concentrations of apo-CS1 in this organelle or to the probe gaining access to CuI made available via recycling of nutrient Cu in the acidic lysosome. It was possible to observe a consistent increase in CS1 fluorescence in neuronal cells exposed to stress. For example, treatment with buthionine sulfoximine decreased cellular glutathione levels and led to enhanced CS1 fluorescence, but the total cellular Cu levels did not correlate with the increased fluorescence. In addition, cells treated with reagents that are known to alter cellular pH homeostasis provided an enhanced fluorescence. Our findings demonstrate that the source of enhanced CS1 fluorescence in Cu-supplemented cells must be interpreted with caution. It may be a consequence of altered cell pH, compromised vesicle maturation, increased CS1 uptake and/or trapping of CS1 in the lysosomal compartment.

 Please wait while we load your content...
Please wait while we load your content...