Electrochemistry and electrogenerated chemiluminescence of thiophene and fluorene oligomers. Benzoyl peroxide as a coreactant for oligomerization of thiophene dimers†
Abstract
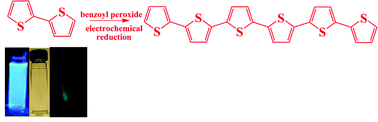
The electrochemical properties of oligomers of thiophene (with number of monomer units, n, from 2 to 12) and fluorene (n = 3 to 7) were investigated. Both sets of oligomers were characterized by the presence of two oxidation and two reduction waves as determined by cyclic voltammetry (CV), with the reversibility of the waves depending on the structural properties of the compounds. The addition or removal of a third electron was found to be difficult relative to the second, a finding shown for conjugated oligomers with chain lengths up to 7 in the case of the fluorenes and up to 12 for the thiophenes. The oligothiophenes showed a larger separation between the electrochemical waves for the same chain length, and also substantial electrogenerated chemiluminescence (ECL) signals, whose intensity increased with oligomer size. In contrast, the ECL intensity of the fluorene oligomers was essentially independent of chain length. The ECL spectra for the thiophene dodecamer were obtained with concentrations as low as 20 pM, a result that reflects a high ECL efficiency, close to that of the well-known ECL standard Ru(bpy)32+. Oligomers were also formed on electrochemical reduction of an appropriately functionalized dimer in the presence of benzoyl peroxide producing a longer wavelength emission (maximum at ∼540 nm) as opposed to the spectrum of the dimer (λem = 390 nm).

 Please wait while we load your content...
Please wait while we load your content...