Pair distribution function-derived mechanism of a single-crystal to disordered to single-crystal transformation in a hemilabile metal–organic framework†
Abstract
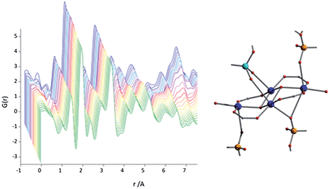
Flexible metal–organic frameworks (MOFs) are materials of great current interest. A small class of MOFs show flexibility driven by reversible bonding rearrangements that lead directly to unusual properties. Cu-SIP-3 is a MOF based on the 5-sulfoisophthalate

 Please wait while we load your content...
Please wait while we load your content...