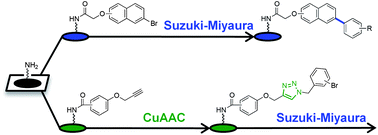
We report the highly efficient syntheses of a series of focused libraries in the small molecule macroarray format using Suzuki–Miyaura and copper-catalyzed azide–alkyne cycloaddition (or “click”) reactions. The libraries were based on stilbene and triazole scaffolds, which are known to have a broad range of biological activities, including quorum-sensing (QS) modulation in bacteria. The library products were generated in parallel on the macroarray in extremely short reaction times (∼10–20 min) and isolated in excellent purities. Biological testing of one macroarray library post-cleavage (ex situ) revealed several potent agonists of the QS receptor, LuxR, in Vibrio fischeri. These synthetic agonists, in contrast to others that we have reported, were only active in the presence of the native QS signal in V. fischeri, which is suggestive of a different mode of activity. Notably, the results presented herein showcase the ready compatibility of the macroarray platform with chemical reactions that are commonly utilized in small molecule probe and drug discovery today. As such, this work serves to expand the utility of the small molecule macroarray as a rapid and operationally straightforward approach toward the synthesis and screening of bioactive agents.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...