Observation and investigation of the uranyl tetrafluoride dianion (UO2F42−) and its solvation complexes with water and acetonitrile†
Abstract
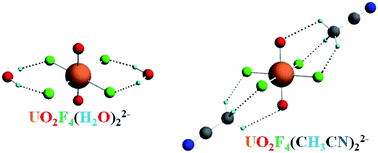
Bare uranyl tetrafluoride (UO2F42−) and its solvation complexes by one and two ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the [O
O bonds in the [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]2+ unit. Each H atom in the
O]2+ unit. Each H atom in the

 Please wait while we load your content...
Please wait while we load your content...