Quinoxalinoneinhibitors of the lectin DC-SIGN†
Abstract
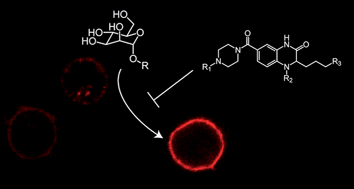
The C-type lectin dendritic cell-specific intercellular adhesion molecule 3–grabbing nonintegrin (DC-SIGN) can serve as a docking site for pathogens on the surface of dendritic cells. Pathogen binding to DC-SIGN can have diverse consequences for the host. DC-SIGN can facilitate HIV-1 dissemination, but the interaction of Mycobacterium tuberculosis with DC-SIGN is important for host immunity. The ability of pathogens to target DC-SIGN provides impetus to identify

 Please wait while we load your content...
Please wait while we load your content...