Oxygen activation in extradiol catecholate dioxygenases – a density functional study†
Abstract
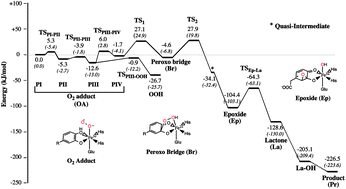
Recent trapping and spectroscopic characterization of an O2 adduct for the non-heme enzyme homoprotocatechuate 2,3-dioxygenase (HPCD) demonstrates it to be a FeIII-superoxo species. This proposal is in direct opposition to the consensus mechanism (J. P. Emerson, E. G. Kovaleva, E. R. Farquhar, J. D. Lipscomb and L. Que, Jr., Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 7347–7352) in which the metal facilitates the transfer of electrons from the substrate to O2 to form the reactive species in the mechanism without changing oxidation state. In this study we performed a detailed analysis of the electronic structure of the O2 adduct for the mutant and native enzymes and the nature of oxygen activation in the reaction mechanism of HPCD using a combination of computational chemistry and theoretical Mössbauer spectroscopy. Our results are in agreement with the available experimental data and demonstrate that even for the native enzyme changes in the metal oxidation state are an important factor in oxygen activation.

 Please wait while we load your content...
Please wait while we load your content...