Lewis base-promoted carbon–carbon sp3–sp3 coupling reactions of α-silyl silylethers†
Abstract
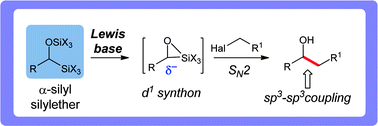
A Lewis base-promoted addition of α-silyl silylethers to primary halides has been developed. This new carbon–carbon sp3–sp3 bond-forming process accesses an unconventional reactivity pattern (d1 synthon) from easily accessible precursors. The strategy accommodates a variety of primary alkyl, allylic and benzylic electrophiles and α-silyl silylethers. These d1 synthons have also been used in the synthesis of cross pinacol and benzil products. Mechanistic studies indicate significant intermolecular silyl group exchange during the reaction.

 Please wait while we load your content...
Please wait while we load your content...