Opening the silole ring: Efficient and specific cleavage of the endo-C(sp2)-Si bond with AcOH/ROH system†
Abstract
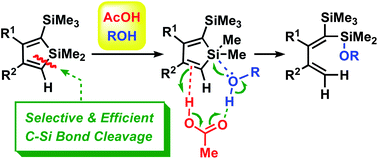
An efficient and specific cleavage of the endo-C(sp2)-Si bond of silole rings was developed. The stable silole ring was effectively opened in an unexpected regioselectivity by using an AcOH/ROH system, in which the AcOH behaved as a

 Please wait while we load your content...
Please wait while we load your content...