Characterization of reaction enthalpy and kinetics in a microscale flow platform†
Abstract
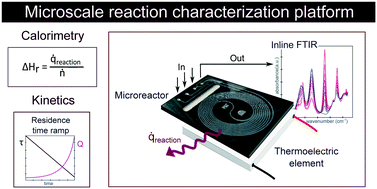
We report an isothermal flow calorimeter for characterization of reaction enthalpy and kinetics. The platform consists of a thermoelectric element and a glass–silicon microreactor to measure heat flux and an inline IR spectrometer to monitor reaction conversion. The thermally insulated assembly is calibrated with a thin film heater placed between the microreactor and the thermoelectric element. Without any reconfiguration of hardware, the setup can also be used to efficiently characterize reaction kinetics in transient flow experiments. We tested the calorimeter with hydrolysis of acetic anhydride as a model reaction. We determined the exothermic reaction enthalpy and the endothermic heat of mixing of the reagent to be −63 ± 3.0 kJ mol−1 and +8.8 ± 2.1 kJ mol−1 respectively, in good agreement with literature values and theoretical predictions. Following calorimetry studies, we investigated reaction kinetics by applying carefully controlled residence time ramps at four different temperatures, and we obtained kinetic rate constants of 0.129 min−1 up to 0.522 min−1 for temperatures between 20 °C and 56.3 °C, also fitting well with data reported in the literature.


 Please wait while we load your content...
Please wait while we load your content...