Carbon material-supported Fe7C3@FeO nanoparticles: a highly efficient catalyst for carbon dioxide reduction with 1-butene†
Abstract
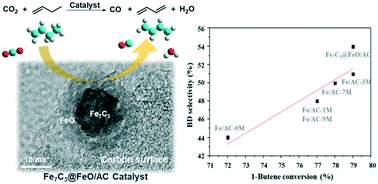
The reaction of carbon dioxide reduction with 1-butene to obtain CO and 1,3-butadiene (BD) is of great importance, but is challenging, especially achieving a highly selective transformation to BD. Herein, we conducted the first synthesis of Fe7C3@FeO nanoparticles supported on activated carbon (AC) which contained acid sites rather than Brønsted acids for carbon dioxide reduction with 1-butene. It was found that this material was very efficient for the reaction; in particular, the BD selectivity could be improved up to 54%. In addition, the effects of the carbon surface chemistry on the catalyst composition, structure and morphology as well as its catalytic properties were investigated. Furthermore, the surface oxygen-containing groups over AC, which provided acid sites, were proved to have played a key role in anchoring and dispersing Fe7C3@FeO nanoparticles, which result in the unique catalytic performance of this material.


 Please wait while we load your content...
Please wait while we load your content...