Integrated leaching–carbonation kinetic model on CO2 mineralization of alkaline solid wastes in a high-gravity rotating packed bed†
Abstract
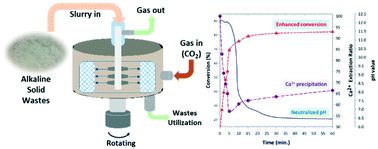
Carbon dioxide (CO2) mineralization by accelerated carbonation using a high-gravity rotating packed bed (HiGee RPB) is a promising process that simultaneously stabilizes industrial alkaline solid wastes and sequestrates CO2 from flue gas. In this study, the carbonation kinetics depicting the CO2 mineralization using different alkaline solid wastes in a HiGee RPB were evaluated based on the surface coverage model. The dynamic changes on Ca2+ concentration during the carbonation process were successfully fitted and described using the Streeter–Phelps formula. The L/S ratio and high-gravity effect on carbonation conversion (δCa), CO2 capture capacity (Ccap), and changes of Ca2+ concentration (CCa2+) were discussed. The kinetic model was validated using experimental data obtained by the batch tests. The results indicated that δCa and reaction constants of different alkaline solid wastes were fitted by the surface coverage model with acceptable R2 values. The proposed kinetic model was used to elucidate the competition between Ca2+ leaching and CaCO3 precipitation during the carbonation. In addition, the liquid side mass transfer rate enhanced by the high-gravity effect was determined. The enhancement factor was used to identify the relationship between the liquid side mass transfer and chemical reaction.


 Please wait while we load your content...
Please wait while we load your content...