Continuous liquid-phase synthesis of nickel phosphide nanoparticles in a helically coiled tube reactor†
Abstract
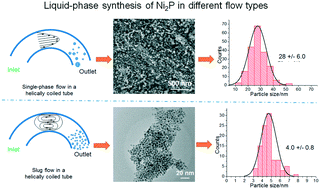
The continuous liquid phase synthesis of nickel phosphide (Ni2P) nanoparticles was studied in a helically coiled tube (HCT) reactor both in single-phase and two-phase slug flows. The reactants were nickel acetylacetonate and tri-n-octylphosphine (TOP) in a 1-octadecane solvent. For the single-phase mode, various parameters such as reaction temperature, residence time, TOP concentration, and P/Ni ratio were studied. It was found that lower temperatures of 320 and 340 °C resulted in the formation of mixed Ni12P5 and Ni2P phases, while a higher temperature of 360 °C gave mostly Ni2P, with particle sizes increasing from 28 to 42 nm. Upon varying the contact time between 88 and 340 s at 360 °C, a likely sequence of reaction involved an amorphous phase that was transformed in parallel to Ni, Ni12P5, and Ni2P. When the flow in the HCT was changed to a two-phase slug flow by using N2 to split the continuous liquid phase into small liquid columns, the product was nearly 100% Ni2P and the particle size was as small as 3–4 nm. This was attributed to the enhanced mass transfer in the small liquid columns of the slug flow that led to higher reaction rates. It is highlighted that the HCT operated in a slug flow is an efficient and continuous method for the fabrication of nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...
