A mathematical model of a slurry reactor for the direct synthesis of hydrogen peroxide†
Abstract
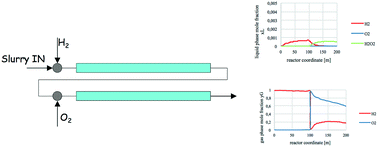
The direct synthesis of hydrogen peroxide represents a green alternative to the conventional large-scale anthraquinone process and offers a significantly economic advantageous way of producing a compound for which the global demand is ever increasing due to its multiple uses. However, the implementation of this process still faces important challenges regarding productivity, selectivity, and safety of this theoretically simple but practically not trivial reaction. In principle, we can smartly implement a process if we deeply know how the system and the reaction proceed. In this perspective, the importance of modeling the process itself becomes clear, and that is the purpose of this study: to develop a mathematical model for the direct synthesis of hydrogen peroxide in a continuous catalytic three-phase reactor. In particular, the fluid dynamic aspects of the system were studied, along with the kinetics and interphase mass exchange. Under our conditions, gas/liquid mass transfer prevailed, thus the reactor worked in a convective mass-transfer regime. Model equations were written and implemented in order to carry out different simulations and to obtain a first dimensioning of the reactor. It should be underlined that the definition of such a model can constitute a step forward and an opening of the doors for the industrial world to implement the direct synthesis of H2O2, ultimately helping make this process an effective industrial production.


 Please wait while we load your content...
Please wait while we load your content...