Mechanism of oligosaccharide synthesis via a mutant GH29 fucosidase†
Abstract
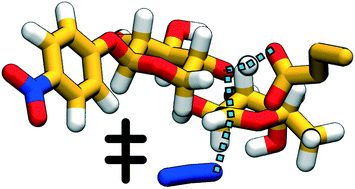
Techniques for synthesis of bespoke oligosaccharides currently lag behind those for other biopolymers such as polypeptides and polynucleotides, in part because of the paucity of satisfactory enzymatic tools to perform the synthetic reactions. One promising avenue of development for this problem is glycoside hydrolase enzymes with mutated nucleophile residues (called glycosynthases), which retain some elements of their native specificity and work with cheaply available substrates. However, the mechanistic underpinnings of this class of enzymes are not yet well-understood, and what few atomistic studies have been conducted have found different reaction pathways. In this paper, we describe the first unbiased computational study of the mechanism of a GH29 glycosynthase enzyme, Thermotoga maritima α-L-fucosidase (TmAfc) D224G. We find a single-step endothermic reaction step with an oxocarbenium-like transition state, demonstrating how stabilization of this transition state structure (which is common to many retaining glycoside hydrolases) can be repurposed in mutant enzymes to perform synthesis instead of hydrolysis. Our results are consistent with previous experimental observations and help both to clarify the mechanism of the existing single-mutant and to provide directions for further engineering of this and other glycosynthases.
- This article is part of the themed collection: Reaction Chemistry & Engineering Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...
