Trimerization and tetramerization of ethylene in continuous gas-phase reaction using a Cr-based supported liquid phase catalyst†
Abstract
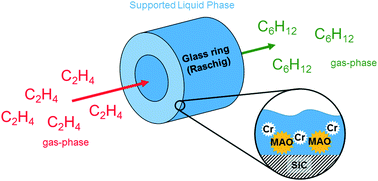
Homogeneous Cr–PNP tri- and tetramerization catalysts were dissolved together with the co-catalyst MAO in the high-boiling, non-polar, aliphatic hydrocarbon perhydro-dibenzyltoluene (H18-DBT). The solution was then dispersed onto different high surface area supports. The resulting solid materials were tested in the gas-phase selective oligomerization of ethylene at 43 °C and pressures between 1 and 14 bar. Low activities were observed at ambient pressure. At ethylene pressures of higher than 3 bar the formed 1-hexene and 1-octene condensed inside the porous system, thereby facilitating the dissolution and redistribution of the Cr–PNP catalyst. Further studies were conducted at pressures up to 35 bar at which a change in the primary reaction selectivity from 1-hexene to 1-octene could be observed. Such a selectivity switch is in accordance with previously reported mechanistic and kinetic studies.


 Please wait while we load your content...
Please wait while we load your content...