Click reaction-aided enzymatic kinetic resolution of secondary alcohols†
Abstract
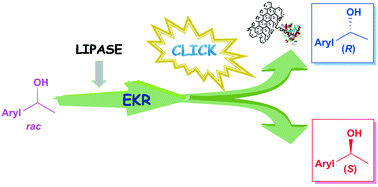
A new and efficient lipase-mediated kinetic resolution (KR)–click-reaction-based procedure is presented for the production of both enantiomers of various 1-(hetero)aromatic ethanols applying enzymatic acylation with 2-(prop-2-yn-1-yloxy)acetyl esters. Click-reaction-based in situ derivatization of the (R)-2-(prop-2-yn-1-yloxy)acetate produced from the reacting enantiomer in the KR step enabled facile and efficient separation of the derivatized (R)-ester and the (S)-alcohol by simple extractions and pH adjustments from the reaction mixture. A mild and efficient enzymatic ethanolysis of the derivatized (R)-ester allowed almost quantitative recovery of the (R)-alcohol without a detectable decrease in enantiopurity. The process could provide, from racemates of various 1-(hetero)aromatic ethanols, almost quantitatively both enantiomers without chromatographic separation of the KR products.


 Please wait while we load your content...
Please wait while we load your content...