Evidence of methane adsorption over Mo2C involving single C–H bond dissociation instead of facile carbon exchange†
Abstract
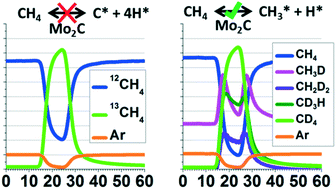
Mo2C catalysts have been widely studied for methane reforming reactions. One of the proposed mechanisms for Mo2C catalysts is a redox type mechanism that includes active participation of the carbide carbon in the reaction. While evidence for this mechanism has been provided by several studies, one of the most surprising results previously reported asserts that a stream of pure methane can undergo significant, facile carbon exchange with the Mo2C catalyst at temperatures above 550 °C. Using pulses of 13CH4, we have found no evidence of carbon exchange between methane and Mo2C, even at 800 °C, in contrast to these previous results. In addition, by using pulses of CD4, we have found evidence of a small degree of dissociative methane adsorption at 800 °C, involving the breaking and reforming of a single methane–hydrogen/deuterium bond. While the present study does not contradict the model of active carbide carbon participation via a redox mechanism in methane reforming reactions, it doesn't support the notion of significant and facile carbon exchange between methane and Mo2C without an oxidant.


 Please wait while we load your content...
Please wait while we load your content...