Environmental and economic assessment of glycerol oxidation to dihydroxyacetone over technical iron zeolite catalysts†
Abstract
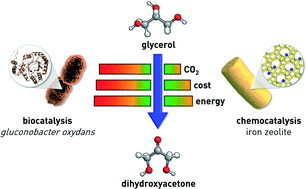
The gas-phase oxidation of glycerol over MFI-type iron zeolite catalysts comprises an attractive technology to prepare dihydroxyacetone with a high productivity, in contrast with the currently practiced biocatalytic system. Herein, we address two crucial aspects in view of a prospective industrial application, i.e., the development of the technical iron zeolite-based catalysts and the environmental and economic assessment of the process by life cycle analysis. Regarding the first task, we show that iron silicalite with the desired structural, acidic and catalytic properties can be prepared by hydrothermal synthesis at the kg-scale and using reagents meeting industrial safety, ecological and cost criteria. The design of suitable mm-sized bodies encompassed the use of shaping methods which minimise the introduction of additional acidity as well as iron clustering and migration from the zeolite to the binder phase. In this respect, silica outperforms kaolin as the binder, pelletisation and polyether-assisted extrusion are superior to water-based extrusion and the calcination and steam activation of the as-crystallised zeolite are preferably done after the forming step. The optimal technical catalyst displays equivalent activity, selectivity and stability to lab-scale pure iron silicalite powder in a 72 h test. From a life cycle perspective, all environmental indicators are drastically improved and the operating cost is halved using a chemocatalytic zeolite-based process for the preparation of high-purity dihydroxyacetone compared to the conventional enzymatic route. This is justified by the high atom economy of the transformation which reduces waste and by the minimisation of the energy input via heat integration and the use of less energy-intensive separation units to purify the substantially more concentrated outlet stream. Since the purity demand for the dihydroxyacetone feedstock to produce lactic acid is lower than that required in the cosmetic and polymer industries, a potential glycerol oxidation process for the former application has an even greater advantage in terms of greenness and cost.

 Please wait while we load your content...
Please wait while we load your content...