meta-Terphenyl linked donor–π–acceptor dyads: intramolecular charge transfer controlled by electron acceptor group tuning†
Abstract
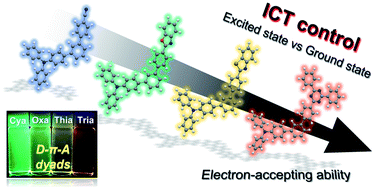
A series of meta-terphenyl linked donor–π–acceptor (D–π–A) dyads were prepared to understand the electronic effects of a meta-terphenyl linker according to the electron-accepting ability change. The energy band gaps of the dyads were controlled by tuning the accepting ability, which resulted in emission colors ranging from blue-green to red. In the Lippert–Mataga plots, intramolecular charge transfer (ICT) behavior was observed, which showed gradually increased ICT characteristics as the accepting ability was increased. On the other hand, in the absorption spectra, a red shift of the ICT transition was observed differently from the electron-accepting ability tendency. Thus, the experimental results show that the ICT is determined by steric hindrance rather than the acceptor ability in the ground state due to the lack of π-conjugation of the terphenyl linker by the electron node in the meta-position, whereas ICT in the excited state is controlled by electron-accepting ability.


 Please wait while we load your content...
Please wait while we load your content...