Hybrid compound based on diethylenetriaminecopper(ii) cations and scarce V-monosubstituted β-octamolybdate as water oxidation catalyst†
Abstract
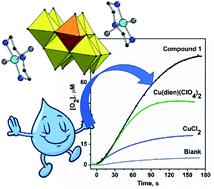
Herein, we report on a new hybrid compound (NH4){[Cu(dien)(H2O)2]2[β-VMo7O26]}·1.5H2O (1), where dien = diethylenetriamine, containing an extremely rare mixed-metal pseudo-octamolybdate cluster. An ex situ EPR spectroscopy provided insights into the formation of paramagnetic species in reaction mixture and in solution of 1. The magneto-structural correlations revealed weak antiferromagnetic exchange interactions between the [Cu(dien)]2+ cations transmitted by intermolecular pathways. The cyclic voltammetry showed the one-electron process associated with the Cu3+/Cu2+ oxidation followed by the multi-electron catalytic wave due to water oxidation with a faradaic yield of 86%. The title compound was thus employed in homogeneous water oxidation catalysis using tris(bipyridine)ruthenium photosensitizer. At pH 8.0, efficiency of the catalytic system attained 0.19 turnovers per second supported by the relatively mild water oxidation overpotential of 0.54 V.


 Please wait while we load your content...
Please wait while we load your content...