Thermal stability and pathways for the oxidation of four 3-phenyl-2-propene compounds†
Abstract
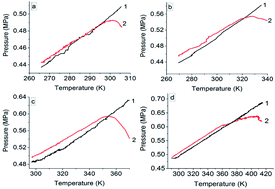
Cinnamaldehyde, cinnamyl alcohol, β-methylstyrene and cinnamic acid are four important biomass 3-phenyl-2-propene compounds. In the field of perfume and organic synthesis, their thermal stability and oxidation pathways deserve attention. This paper reports a new attempt to investigate the thermal stability and reactivity by a custom-designed mini closed pressure vessel test (MCPVT). The pressure and temperature behaviors were measured by MCPVT under nitrogen and oxygen atmosphere. The temperature of initial oxygen absorption (Ta) and rapid oxidation (TR) were calculated. The results showed that four 3-phenyl-2-propene compounds were stable under nitrogen atmosphere. The Ta of cinnamaldehyde, cinnamyl alcohol, β-methylstyrene, and cinnamic acid was 271.25 K, 292.375 K, 323.125 K, and 363.875 K, and their TR was 301.125 K, 332.75 K, 357.91 K, and 385.375 K, respectively. The oxidation reactivity order was derived to be cinnamaldehyde > cinnamyl alcohol > β-methylstyrene > cinnamic acid. The oxidation kinetics were determined using n versus time (n–t) plots, which showed a second-order reaction. Peroxide was determined by iodimetry, and the oxidation products were analyzed by gas chromatography-mass spectrometry (GC-MS). The results showed that the peroxide value of cinnamaldehyde, cinnamyl alcohol, β-methylstyrene, and cinnamic acid reached 18.88, 15.07, 9.62, and 4.24 mmol kg−1 at 373 K for 6 h, respectively. The common oxidation products of four 3-phenyl-2-propene compounds were benzaldehyde, benzoic acid, and epoxide, which resulted from the carbon–carbon double bond oxidation. The substituents' oxidation products were obtained from the oxidation of cinnamaldehyde, cinnamyl alcohol, and β-methylstyrene. In particular, the difference is that no oxidation products of the carboxyl group of cinnamic acid were detected. The common oxidation products of the four 3-phenyl-2-propene compounds were benzaldehyde, benzoic acid, and epoxide, which resulted from the carbon–carbon double bond oxidation. The substituents' oxidation products were caught in the oxidation of cinnamaldehyde, cinnamyl alcohol, and β-methylstyrene. In particular, the difference is that no oxidation products of the carboxyl group of cinnamic acid were detected. According to the complex oxidation products, important insights into the oxidation pathways were provided.


 Please wait while we load your content...
Please wait while we load your content...