Iron porphyrin-catalyzed N-trifluoroethylation of anilines with 2,2,2-trifluoroethylamine hydrochloride in aqueous solution†
Abstract
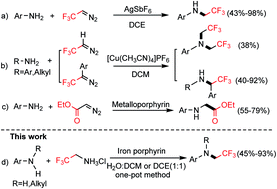
An iron porphyrin-catalyzed N-trifluoroethylation of anilines has been developed with 2,2,2-trifluoroethylamine hydrochloride as the fluorine source. This one-pot N–H insertion reaction is conducted via cascade diazotization/N-trifluoroethylation reactions. The developed transformation can afford a wide range of N-trifluoroethylated anilines in good yields using readily available primary amines and secondary anilines as starting materials.


 Please wait while we load your content...
Please wait while we load your content...