Boron-catalyzed dehydrative allylation of 1,3-diketones and β-ketone esters with 1,3-diarylallyl alcohols in water†
Abstract
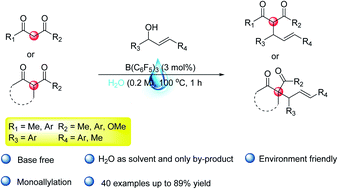
A metal-free catalytic allylation with atom economy and green environment friendly was developed. Allylic alcohols could be directly dehydrated in water by B(C6F5)3, without using any base additives. The reaction can afford the corresponding monoallylated product in moderate to high yield and has been performed on a gram-scale, and a quaternary carbon center can be constructed for the active methine compounds of 1,3-diketones or β-ketone esters in this process. The product can be further converted, such as the synthesis of tetra-substituted pyrazole compounds, or 1,4-dienes and functionalized dihydropyrans.


 Please wait while we load your content...
Please wait while we load your content...